Understanding Chemical Equilibrium: A Comprehensive Guide
What is Chemical Equilibrium?
In a chemical reaction, reactants are transformed into products. For many reactions, this process is reversible, meaning that products can also revert back into reactants. When the rate at which reactants turn into products equals the rate at which products revert to reactants, the system is said to be at equilibrium. At this point, the concentrations of all reactants and products remain constant, although both reactions continue to occur.
Dynamic Nature of Equilibrium
It’s crucial to understand that chemical equilibrium is dynamic, not static. This means that even though the macroscopic properties (like concentration) remain unchanged, the microscopic processes of the forward and reverse reactions continue to happen.
Chemical Reaction in Action:
Picture a reaction between molecules, like mixing vinegar (acetic acid) and baking soda (sodium bicarbonate). They bump into each other, and if they have the right fit (collision theory), they can react to form new products (carbon dioxide and water).
The Slowdown:
As the reaction progresses, there are fewer reactant molecules available to bump and react. This slows down the rate of the forward reaction (vinegar and baking soda forming products).
The Comeback:
But here’s the twist! Those new products can also bump into each other and react in the reverse direction (carbon dioxide and water reforming vinegar and baking soda).
The Balancing Act:
Eventually, the rates of the forward and reverse reactions become equal. This doesn’t mean they stop completely,but rather that they happen at the same speed. We call this state chemical equilibrium.
Dynamic, Not Static:
Even at equilibrium, the reactions don’t completely freeze. It’s like a tug-of-war where both sides are constantly pulling, but neither gains ground. Individual molecules are constantly reacting in both directions, but the overall amounts of reactants and products remain stable.
Factors Affecting the Balance:
We can influence this balance by changing certain conditions. Adding more vinegar (reactant) will push the reaction forward initially, but eventually, the new equilibrium will be established with more products. Similarly, changing temperature or pressure can also affect the equilibrium position.
So, chemical equilibrium is a dynamic state where opposing reactions (forward and reverse) occur at the same rate,resulting in a stable concentration of reactants and products. It’s all about the constant dance of molecules reacting in both directions!
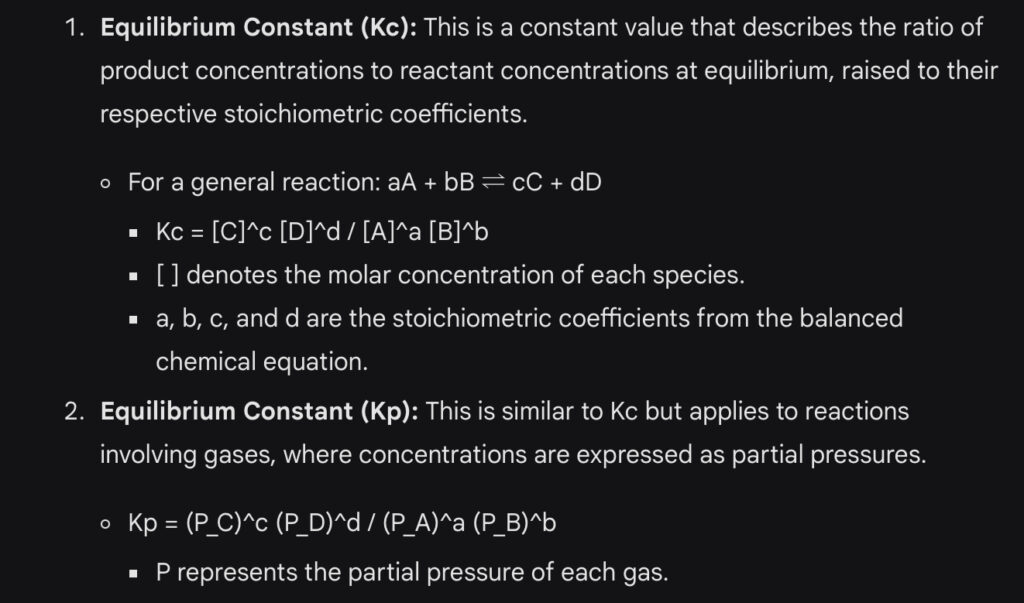
Important Formulas of Chemical Equilibrium

Factors Affecting Equilibrium
Several factors can affect the position of equilibrium in a chemical reaction:
- Concentration Changes: Adding or removing reactants or products can shift the equilibrium position according to Le Chatelier’s Principle, which states that a system at equilibrium will adjust to counteract any changes imposed on it.
- Temperature Changes: Increasing or decreasing temperature can affect the rates of the forward and reverse reactions differently, thereby shifting the equilibrium position. For exothermic reactions, an increase in temperature shifts the equilibrium to favor the reactants, while for endothermic reactions, it favors the products.
- Pressure Changes: For reactions involving gases, changing the pressure by altering the volume of the reaction vessel can shift the equilibrium. An increase in pressure will shift the equilibrium towards the side with fewer gas molecules, while a decrease in pressure favors the side with more gas molecules.
- Catalysts: While catalysts speed up the rate at which equilibrium is reached, they do not affect the position of equilibrium. They equally accelerate both the forward and reverse reactions.
Examples of Chemical Equilibrium
The Haber Process
The Haber process for synthesizing ammonia from nitrogen and hydrogen is a classic example:
At equilibrium, the rate of formation of ammonia equals the rate at which it decomposes back into nitrogen and hydrogen.
2. Acetic Acid Dissociation
The dissociation of acetic acid in water is another example
Le Chatelier’s Principle in Action
Example: Consider the equilibrium involving dinitrogen tetroxide (N2O4) and nitrogen dioxide (NO2):
If additional NO2 is added to the system, the principle predicts that the equilibrium will shift to the left to counteract the change, producing more N2O4 and consuming some of the added NO2.
Practical Applications of Chemical Equilibrium
- Industrial Processes: Many industrial processes, like the Haber process, rely on manipulating equilibrium conditions to maximize the yield of desired products.
- Pharmaceuticals: Understanding equilibrium is crucial in the formulation of drugs, especially in controlling the concentrations of active ingredients.
- Environmental Science: Equilibrium principles help in understanding and predicting the behavior of pollutants and their interactions in the environment.
Conclusion
Chemical equilibrium is a cornerstone of chemical understanding, revealing the balance point in reversible reactions. By comprehending how equilibrium constants, Le Chatelier’s Principle, and various factors influence this balance, chemists can predict and manipulate reaction conditions to optimize outcomes in diverse fields, from industrial manufacturing to environmental conservation. This dynamic state of balance showcases the intricate dance of reactants and products, underpinning much of the observable behavior in chemical systems.
In chemistry, some reactions don’t use up all the starting ingredients. Chemical equilibrium is when the speed of the “cake-making reaction” (forward reaction) equals the speed of the “cake-unmaking reaction” (backward reaction). At this point, even though both reactions are still happening, the amounts of reactants and products stay the same, like a balanced seesaw.
Think of it like kids playing tag. Some kids are tagging others (reacting), but eventually, tired kids who were “it” become regular players again (reacting back). Equilibrium is when there’s a constant number of kids being “it” and regular players,not one side completely taking over.
Understanding equilibrium is important in many areas. It helps explain how medicines work in our bodies, how pollutants behave in the environment, and even how efficiently factories produce things. So, the next time you see a chemical reaction, remember it might be a balancing act, not a one-way street!

